Apoptosis and its health giving benefits

Apoptosis
The ability to recycle a cell back into CO2 and water is the naturally cellular process called apoptosis. Cells have this ability and they do this when they accumulate too much damage within their proteins, lipids, and DNA and RNA. The biological signals that stimulate cellular death are many and varied but the downstream consequences of those signals involve the release of cytochrome c from the mitochondria. The cascade of events includes activation of caspase enzymes and the cellular deconstruction process leads to the release of apoptotic bodies. This is well outlined in the scientific literature.
What I postulate is the following. Because the mitochondria is no longer functioning during apoptosis it is assummed that ATP is not being generated. If there is no ATP then how does the cell generate energy to break the bonds in DNA, RNA, proteins and carbohydrates. You could think enzymes are doing the work to release the amino acids, nucleosides and sugars, however, the genetic material is broken down so how do the genes get expressed to produce the enzymes needed for apoptosis. You could say well they are there all along and just activated. I consider the apoptosis process to be linked directly to photo-Fenton chemistry and the generation of hydroxyl radicals. If the mitochondria is not making ATP because cytochrome c has been released. Then, SOD could take superoxide to produce hydrogen peroxide which provides the chemical energy for Fenton chemistry in the apoptotic cell. The reactions are shown below in relation to the benzene ring and how this can be broken down into CO2 and water. If radicals do chemical damage to cells as postulated as the cause of disease by the health industry, then antioxidant would be helpful. However, this natural cell death process that recycles the biological molecules in cells back into CO2 and water uses hydroxyl radicals and therefore stopping the hydroxyl radical with an antioxidant would prevent apoptosis. I believe the logic behind this biological recycling system is evident in the outcomes of using the product I make. OH BEE HAVE empowering healing supports a persons healing by removing damaged cells from a persons body.

So by supporting the apoptosis processes in cell biology you can support your cellular wellbeing. This is a complete 180 degree position that I have taken. If you consider the burden of western disease and how it has increased over the years. You will be able to draw a correlation between Westerized disease and antioxidant use.
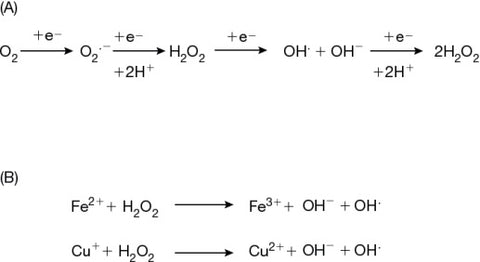
Fenton chemistry and the hydroxyl radical is the most powerful oxidant but it also creates molecules in this process.
(1) Fe2+ + H2O2 → Fe3+ + HO• + OH–
(2) Fe3+ + H2O2 → Fe2+ + HOO• + H+
Iron(II) is oxidized by hydrogen peroxide to iron(III), forming a hydroxyl radical and a hydroxide ion in the process. Iron(III) is then reduced back to iron(II) by another molecule of hydrogen peroxide, forming a hydroperoxyl radical and a proton. The net effect is a disproportionation of hydrogen peroxide to create two different oxygen-radical species, with water (H+ + OH–) as a byproduct.
Both copper and iron can form hydroxyl radicals.
The Why do radicals have beneficial properties in our bodies?
The radicals in your body are also involved in destroying pathogens and viruses in a process that is known as the oxidative burst. See the information below.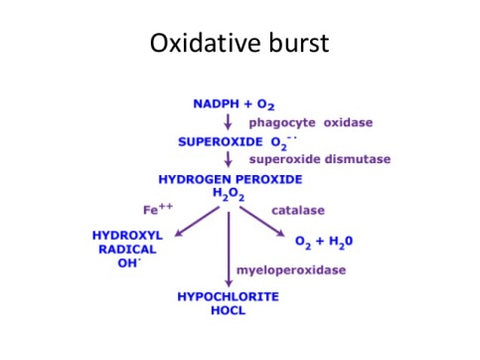
This oxidative burst process produces anti-bacterial and antiviral properties and this is due to radicals in a special vescile called the phagosome.
Radical role in quantum entanglement
Radicals also have an important role in quantum entanglement. How is light held together in a coherent orderly fashion so that it works together to create structures without falling apart and without doing something that it should not do. As shown in this example where radicals have been used to generate a quantum entangled solution which responds coherently at different frequencies of light (UVA) to create biological structures.
Redox Biology Volume 10, December 2016, Pages 233-242. Hydroxytyrosol inhibits hydrogen peroxide-induced apoptotic signaling via labile iron chelation. https://doi.org/10.1016/j.redox.2016.10.006
Yes, radicals are important in biology. Anti-oxidants are also important. They capture the radicals to create molecules that the body uses. The hydroxyl group on neurotransmitters serotonin and dopamine are created by hydroxylation and the enzyme has Fe2+ at the active site. Radical chemistry creates OH* and creates the exact same structure but it does it much faster 1 billionth of a second. So has the potential to make 1,000,000,000 hydroxylation events in 1 second. More control by the enzyme creating a contained environment for the hydroxylation event to occur only on the aromatic ring but the reaction results in the same outcome produced by an enzyme or by hydroxyl radical generation. The evolutionary process started first in physics.
Cancer and radicals and their role in apoptosis
Cancer formation appears to be an adaptive response to stopping the normal radical chemistry that occurs in a cell undergoing apoptosis and generating CO2 and water from the dying cell as shown below.
Is cancer a result of a cell failing to undergo apoptosis (pre-programmed cell death)? If a cell fails to die and in the process accumulates enough changes in the cell to allow adaptation to occur by accumulating sufficient genetic modification to create a new cancer cell that has the ability to adapt to its environment by changing which genes are expressed.
Hydroxyl radical mediates cisplatin-induced apoptosis in human hair follicle dermal papilla cells and keratinocytes through Bcl-2-dependent mechanism. Apoptosis. 2011 Aug;16(8):769-82. doi: 10.1007/s10495-011-0609-x. by S Luanpitpong - 2011. Studies using specific ROS scavengers further showed that hydroxyl radical, but not hydrogen peroxide or superoxide anion, is the primary oxidative species responsible for the apoptotic effect of cisplatin. Electron spin resonance studies confirmed the formation of hydroxyl radicals induced by cisplatin.

Figure 8: Radicals processes in cell apoptosis (pre-programmed cell death) and production of apoptotic bodies
Apoptosis requires hydroxyl radicals to breakdown the biomaterials into CO2 and Water. Cellular damage occurs to convert biological molecules into CO2 and H2O. So radicals are important in biological signaling as well as cellular recycling events including apoptosis. Without apoptosis would cellular proliferation diseases be produced that leads to cancer formation?
What if we have got it wrong and radicals are not bad but important in health and vitality and by using purified anti-oxidants we are stopping he very free radicals in our bodies that are essential for apoptosis? What if we are turning off our natural apoptosis system of cellular death and regeneration and inducing proliferation diseases that initiates cancer formation?

Figure 16: Hydrogen peroxide signaling and apoptosis
The production of hydrogen peroxide can occur in the mitochondria by the production of superoxide and the enzyme superoxide dismutase. The pores in the mitochondria may enhance H2O2 release into the cell and Fenton chemistry is then responsible for the production of hydroxyl radicals and the ultimate compound responsible for the conversion of the cell back into CO2 and water.
So from the above information it should start to become obvious that radicals and oxidative compounds including superoxide, nitric oxide, hydrogen peroxide and the hydroxyl radical are key functional molecules in cellular signaling and apoptosis. They appear to provide key mediators of biological processes including apoptosis, stem cell regeneration, neurotransmitter function.

What if the oxidative stress response was an adaption to change in the earths atmospheric conditions? The changes in the gas composition on our planet which went from an anoxic environment (without oxygen) to an oxygenated environment, before photosynthesis became widespread? This would suggest that anoxic biochemical pathways that did not require free oxygen as the terminal electron acceptor to have evolved before mitochondrial ATP based energy generation biochemical systems. The glyoxylase pathway would be a good example of the type of chemistry involved in early energy generation. The currently held widespread thinking is that the glyoxalase system is the main catabolic route for methylglyoxal, a non-enzymatic glycolytic byproduct with toxic and mutagenic effects. As this compound is highly reactive towards lysine, arginine and cysteine residues via radical chemistry it appears to regulate thiol oxidation states within the cell through reaction with glutathione (GSH).
It appears that stem cells in our bodies are located in environments that are low in oxygen and their evolutionary adaption and changes (differentiation into different cell phenotypes) occurs with the help of ROS (reactive oxygen species). The stem cell typically will not have functional mitochondria and this makes sense as the cellular environment with its low oxygen level means there is insufficient oxygen for mitochondria to function properly. So how do stem cells produce energy? My hypothesis is that they generate energy from ROS via radicals. So if we stop stem cell differentiation that requires ROS then anti-oxidants are impacting the regeneration processes that are occurring in our bodies. This suggests that antioxidants may accelerate aging in our bodies and that radicals are important for biological recycling and maintaining health and vitality.
Nitric oxide
Nitric oxide is another critical signaling radical in our bodies. It is important in cardiovascular health and sexual function. The enzymes responsible for activity require iron and zinc for activity.

Nitric oxide is important radical in vascular health and vasodilation.

Just think what would happen without the right signaling in the body. Dis-ease!
Balance and homeostatis
There is obviously a balance between health and disease. What if the pendulum has swung too far towards the anti-oxidant side of the pendulum? Low energy, low vitality, an inability to think on your feet and adapt quickly.

There is a potential misunderstanding about antioxidants, yes they create stability but radicals enable biological recycling through apoptosis. Does the body obtain harmony and balance by doing the opposite to what you add externally. So if you add antioxidants externally then your body does not need to produce as much of its own anti-oxidants. Also, your body will also produce more oxidants to compensate for the antioxidants used. The bodies oxidant system which is involved in the following biological processes. 1) Hydroxyl radical OH* apoptosis or pre-programmed cell death, 2) Nitric oxide NO* in blood pressure and sexual function, 3) hydrogen peroxide as a signaling molecule in many biological pathways.
Balance Hypothesis
If antioxidants are added in excess in the diet then there are many biological processes are likely to be effected. Do we know where these antioxidants are going in our bodies? They love coordinating with metals in enzymes and binding to proteins. The can act as inhibitors. Yes they can. Do we need these enzyme inhibitors in our diets?
From a Darwinian perspective the plants have developed a defense system which impacts on the health and well-being of animals that feed on the plants. Plants and animals have evolved in a dynamic relationship at many levels they need one another and are tied into a symbiotic relationship exchanging gases CO2 and O2. However, when highly purified antioxidants that are added into a humans diet which capture radicals (singlet electron compounds) appear to be able to inhibit many biochemical pathways. Therefore, impacting on human health and well being. The more anti-oxidants added the more stable the cell will become that could result in a continuous accumulation in damage without an ability to undergo apoptosis. The body will reduce the production of its own anti-oxidant systems and enzymes because it can use the external sourced of anti-oxidants. To counter balance the effects of external anti-oxidants the body may also generate more free radicals to compensate for the anti-oxidants.
So the initial use of anti-oxidants will produce an improvement due to the compensation and changes that occur within the body to address the use of additional anti-oxidants in the diet. This suggest that purified anti-oxidants used in the diet are initially beneficial but then are detrimental to ones health and well-being and have the potential to lead to the formation of disease.
On the other hand if additional oxidants are added to the body then the reverse will be observed. The body will produce more anti-oxidant enzyme to compensate and the body will have higher energy levels and the person will have greater vitality. Therefore, there is a significant body of evidence for the health giving properties of radicals and their involvement in many biochemical pathways in your body. The ability of radicals to generate energy via electron transport, radicals ability to hydroxylate neurotransmitters and their involvement in various functional biochemical pathways suggests we need to reconsider the widespread use of anti-oxidants as an evidence based approach for maintaining health and well-being. Human disease rates are on the rise. What is the underlying reason why? Time for a rethink in human health and well-being.
